In recent years, the rapid increase in antibiotic resistance has made it challenging to treat bacterial infections, raising the risk of disease spread, severe illnesses, and death. Once hailed as miracle drugs, antibiotics are losing their effectiveness due to the continuous evolution of bacteria, prompting scientists to seek solutions.
CRISPR for Antibiotic Resistance
Biotechnology firms are exploring different treatment strategies to address antibiotic resistance. Locus Biosciences, based in North Carolina, aims to offer a new treatment method by equipping bacteriophages—viruses that target bacteria—with CRISPR technology.
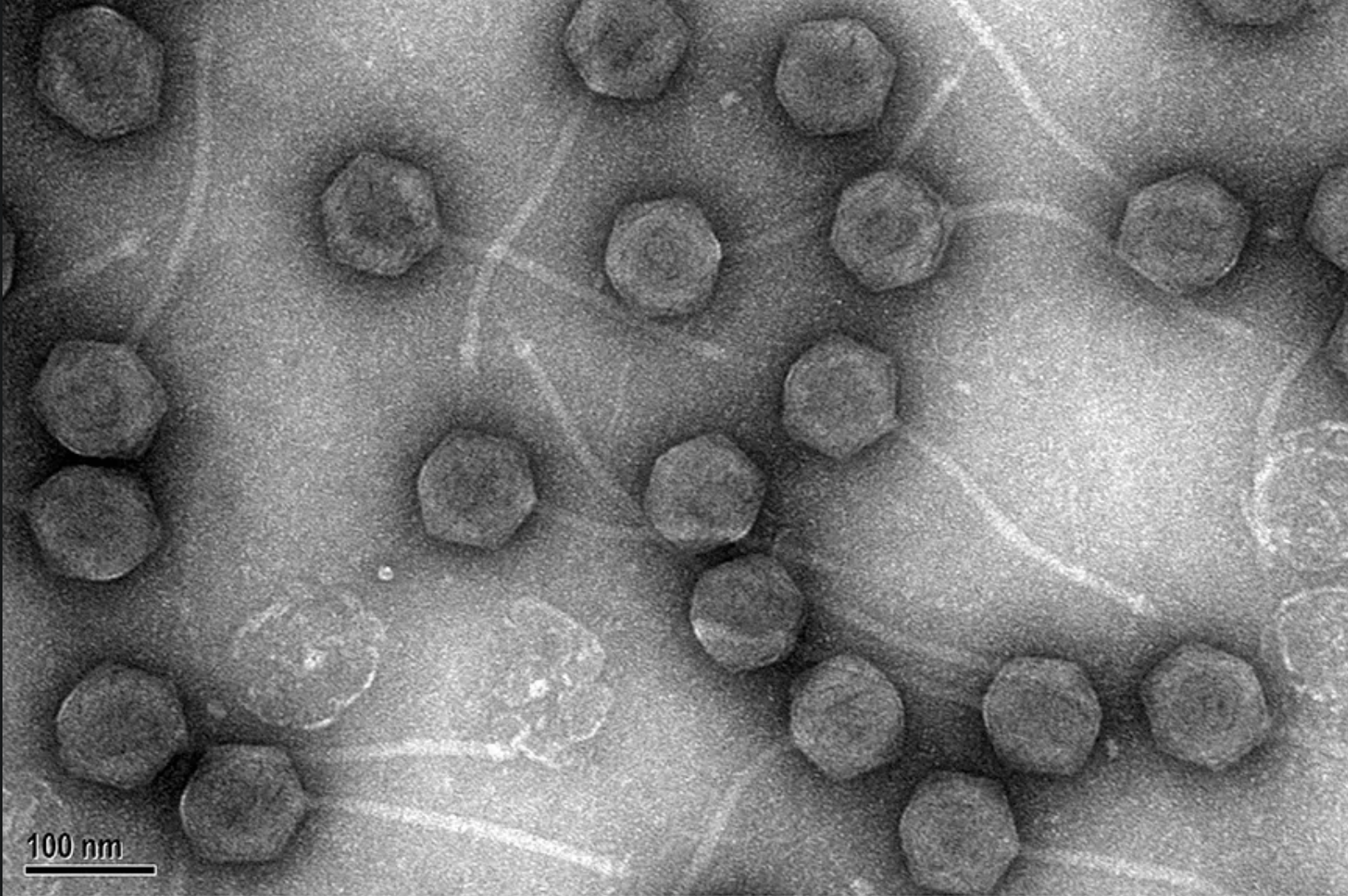
Bacteriophages are viruses that naturally infect and kill bacteria. Locus Biosciences intends to enhance these viruses’ killing ability using CRISPR gene-editing technology. The company’s new treatment approach is being tested against urinary tract infections caused by a bacterium known as E. coli.
Results from a small-scale study published in August indicate that this experimental treatment shows promise, though larger-scale trials are needed.
In the clinical trial of this treatment, 16 women received a combination of phage cocktail and the widely used antibiotic Bactrim over three days. Just four hours after the initial treatment, the levels of E. coli in the patients’ urine rapidly decreased and remained low by the end of the 10-day study period. Fourteen out of the 16 women managed to fully recover from the infection by the end of the treatment.
Findings published in The Lancet Infectious Diseases on August 9 show that the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority is involved in the research.
While bacteriophage therapy is not yet commercially approved in the U.S., it can be applied in certain special cases with FDA approval. The personalized nature of phage therapy makes widespread application challenging.
Future clinical trials will expand to include control groups to better understand the treatment’s effectiveness. The new research will compare a control group receiving only Bactrim with a trial group receiving both Bactrim and the phage cocktail, and plans to involve 288 participants.














